Instruction leaflet for medical use of the pharmaceutical product
Septaphage® (Bacteriophagum Intestinalis Liquidum) 20 ml sterile solution for internal use
Septaphage® (Bacteriophagum Intestinalis) 0.13 g tablet for oral use
Trade name
Septaphage®
International proprietary name Bacteriophagum Intestinalis Description
Sterile solution
Various intensity yellow, transparent solution with specific taste.
Tablet
White, grayish tablets.
Composition:
Active ingredient
1ml of fluidy product contains phagolysates sterile filtration mixture of bacteria: Shigella Flexneri
1,2,3,4,6 serogroup; Shigella Sonnei; Salmonella: paratyphi A, B, Typhimurium, Choleraesuis,
Oranienburg, Enteritidis; Enteropathogenic E.coli – etiologically important serogroups of enteral diseases; Proteus: Proteus (vulgaris, mirabilis); Staphylococcus; Pseudomonas; Enterococcus.
1 tablet contains sterile filtration concentration of liophylically dried phagolysates of bacterias:
Shigella Flexneri 1,2,3,4,6 serogroup; Shigella Sonnei; Salmonella: paratyphi A, B, Typhimurium, Choleraesuis, Oranienburg, Enteritidis; Enteropathogenic E.coli – etiologically important serogroups of enteral diseases; Proteus: Proteus (vulgaris, mirabilis); Staphylococcus; Pseudomonas; Enterococcus.
Quantitative composition
1 ml of fluidy product and 1 tablet contain specific phages:
Shigella: Flexneri 1,2,3,4,6 serogroup B; Sonnei.
Salmonella: paratyphi A, B, Typhimurium, Choleraesuis, Oranienburg, Enteritidis
E.coli: etiologically important serogroups of enteral diseases;
Proteus (vulgaris, mirabilis)
Staphylococcus Pseudomonas Enterococcus
in not less than 105 each one.
Pharmaceutical form
20 ml sterile solution for internal use: for oral and for rectal use (Per os/ Per rectum).
0.13g tablet for oral use.
Pharmacotherapeutic group: Specific antibacterial preparation.
ATC code: J01XX
Pharmacological activity: Bacteriophage is a specific virus of bacteria which is adsorbed on the membrane of cell of homological bacteria , destroying its integrity penetrates into cell, propagates itself and causes its lysis.
Septaphage® contains only selected virulent phages of bacteria (Shigella, Salmonella, E. coli, Proteus,
Staphylococcus, Pseudomonas, Enterococcus) conditioning high activity and effectiveness of the
preparation.
Despite of the way of administration preparations of Bacteriophage are rapidly penetrated in blood and lymph and thus easily reach to the disease. It is secreted mainly in kidneys and in gastrointestinal tract. Therapeutic indications: Septaphage® is used for treatment and prophylaxis of the diseases caused by bacteria (Shigella, Salmonella, E.coli, Proteus, Staphylococcus, Pseudomonas, Enterococcus) or diseases caused by unity of them such as: shigellosis, typhoid, paratyphoid, dysbacteriosis
enterocolitis, infectious colitis, dyspepsia; foodborne infections and other bacterial diarrheas; Also discomfort of gastrointestinal tract, in patients of all age and high risk groups;
Contra-indication: Increased sensitivity to any components of the vial content of the product.
Special precautions: There are no special precaution measurements when using the preparation. The
sterile syringe should be used for taking out the content from the vial of sterile solution form of the preparation (in case when using less than 20ml of the solution). In case if the integrity of the vial is destroyed the content of the vial should be used within 24 hours after opening when storing conditions are completely protected.
It’s not allowed to take preparation when the content is grown turbid.
Interaction with other pharmaceutical products: Incompatibility of the preparation with other
pharmaceutical products is not ascertained. The preparation is possible to be used in complex treatment with other pharmaceutical products, including antibacterial preparations (antibiotics).
Pregnancy and lactation: The product is allowed to be used in pregnancy and lactation.
Driving and machinery operation: Using of the preparation doesn’t influence on ability of driving nad machinery operations.
Keep in a place unreachable for children.
Dosage, rule and way of administration: One of the important premises of phage therapy effectiveness
is the definition of sensitivity of microorganism to phage (to phages included in the preparation) defiant the disease. The preparation is more effective while using in early stage of the disease.
Septaphage® is prescribed by physician for using per os/per rectum.
Septaphage® is indicated per os in the first day of the disease when revealing first symptoms, prior to 1 hour before meal 2-3 times per day during 5-10 days. While treatment using the preparation per os for achieving more effectiveness of the course the fluid form of Septaphage is indicated also per rectum to be administered by micro enema once per day at night before sleep after gastric action.
Recommended dosage scheme for sterile solution for treatment of intestine infections :
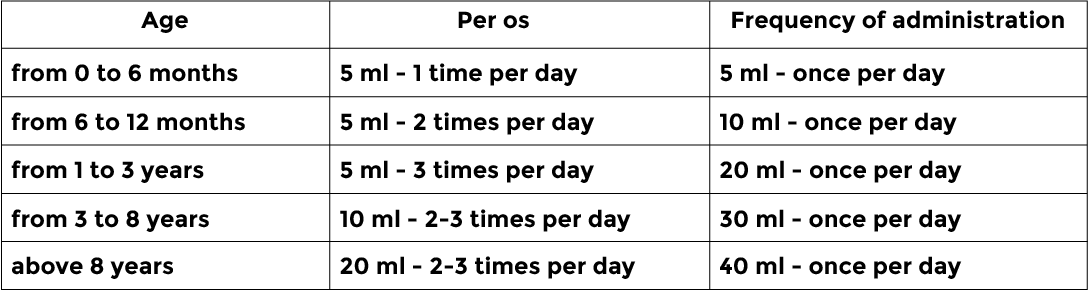
Recommended dosage scheme for tablet form for treatment of intestine infections :

Prophylaxis of intestinal infections: For avoiding intestinal infections the preparation is indicated 3 times per week according to the recommended scheme:

Over dosage: There are no any information about over dosage of the preparation.
Adverse reactions: The preparation is nontoxic. There are no data evident about any side effects or harm influence on human body after administration of the product.
Form and package of the product
Sterile solution
The product is produced in vials of 20 ml medical glass. The package contains 20 ml 4 vials of the
preparation together with instruction leaflet for use.
Tablet
The package contains tablet N30 of the product placed in polyethylene bottle and instruction leaflet for
use.
Storage and transportation terms
Sterile solution
Keep in a dry place, away from light at a temperature 2°С – 25°С.
Tablet
Keep in a dry place away from light in a closed bottle at not more than 25°С temperature.
Shelf life
The product is valid during 2 years since the date of manufacture if proper storage conditions are
protected. The product shouldn’t be used after the date of expiration.
Release from drug stores
The III pharmaceutical product group (released without prescription).
Manufacturer: JSC “Biochimpharm”, address: 3, Gotua str., 0160, Tbilisi, Georgia
Tel: +995 32 2244778; Fax: +995 32 2380895;
Web-site: www.biochimpharm.com
E-mail: info@biochimpharm.com





